One morning in the summer of 1961, hundreds of crazed birds attacked the seaside town of Capitola, California. The birds "cried like babies" as they dove into streetlamps, crashed through glass windows, and attacked people on the ground. Most of the birds were sooty shearwaters, a normally nonaggressive species that feeds on small fish and comes ashore only to breed.
The incident fascinated Alfred Hitchcock, who frequently vacationed in nearby Santa Cruz. He included newspaper clippings about the Capitola attack in his studio proposal for The Birds, which appeared in cinemas two years later.
In the winter of 1987, the agent that is now believed to be responsible for the Capitola incident struck on the opposite shore of the continent. This time, it struck higher on the food chain. Over a hundred people became extremely ill within hours after dining on cultured blue mussels in restaurants around Prince Edward Island in Canada. It quickly became apparent that this was no ordinary outbreak of food poisoning. Vomiting, cramps, diarrhea, and incapacitating headaches were followed by confusion, loss of memory, disorientation, and (in severe cases) seizures and coma. A few exhibited emotional volatility, with uncontrolled crying or aggressiveness. Three elderly victims died. [Perl].
A tragic symptom of poisoning was the destruction of short term memory in about one quarter of the survivors. They could remember nothing that happened after the poisoning. Some were unable to recognize their surroundings or relatives. They could learn no new facts or skills. The most severely affected lost memories several years old. For twelve of the victims, the loss of short term memory was permanent.
The mysterious syndrome was called "amnesic shellfish poisoning". This sort of neurological damage due to food poisoning had never been encountered before. To prevent further injury and loss of life it was imperative that the toxic agent be isolated and identified as quickly as possible. A team of marine biologists and chemists was assembled by Canada's Department of Fisheries and Oceans (DFO) to work on the problem.
But quick resolution of the mystery was unlikely. An initial screening of the sample for known bacterial and viral pathogens revealed nothing. Tests for heavy metals, pesticides, and PCBs also were negative. The mussel samples were extremely complex, containing thousands of different chemical compounds. How can one component be isolated from a such a complex mixture, without knowing anything about its physical or chemical properties?  How do you find a needle in a haystack, when you've never seen a needle before?
How do you find a needle in a haystack, when you've never seen a needle before?
Suppose a test could be devised for the presence of the needle in a haystack. The haystack could be divided in half, and the half that tested negative for the needle could be discarded. Repeating this divide-and-discard process over and over again should eventually result in a pile with only one thing left: the needle.
That was the strategy the researchers used to isolate the toxin. A reliable but gruesome biological test was developed. Injection of a small amount of the sample into mice produced a very distinctive neurological reaction if the toxin was present: the mice involuntarily scratched their shoulders with their hind legs. [Teitelbaum]
Standard physical methods for separating complex mixtures were applied to the poisoned mussel samples. At the same time, uncontaminated mussels were subjected to the same separations, to allow the analysts to compare fractions. Any differences in spectra or chromatograms between the control and toxic samples might be valuable clues in the search for the toxic agent. Mice were exposed to each fraction of the separation. Fractions found to be toxic were retained for further analysis. The others were discarded. If chromatograms and spectra indicated that the toxic fraction was still a complex mixture, another separation technique was applied (see Figure 1).

Figure 1. General strategy for isolation of the toxin responsible for amnesic shellfish poisoning. Based on a diagram by M. Quilliam and J. L. C. Wright (Analytical Chemistry, 61, 1054 (1989)).
Separation by solubility and volatility
Most drugs and poisons are either fat soluble or water soluble, so a logical first step in the isolation was solvent extraction . To prevent potential decomposition of the compound by heat or harsh solvents, ground mussel samples were extracted at room temperature with aqueous methanol, a mild solvent. The extraction was inefficient but successful: mice had the same neurological reaction to the methanol extract that they had to the original mussel samples.
. To prevent potential decomposition of the compound by heat or harsh solvents, ground mussel samples were extracted at room temperature with aqueous methanol, a mild solvent. The extraction was inefficient but successful: mice had the same neurological reaction to the methanol extract that they had to the original mussel samples.
The extract was concentrated by evaporation. The vapor was not toxic, but the residue after evaporation was. The poison apparently was nonvolatile, which could indicate a high molecular weight compound, or a compound that ionized in solution.
A second extraction was performed by shaking the concentrated extract with a mixture of a nonpolar solvent (dichloromethane and water, which is polar. The two solvents don't mix; they settle into two easy separable layers.
and water, which is polar. The two solvents don't mix; they settle into two easy separable layers.
The dichloromethane fractions for the toxic mussels contained several colored substances absent in the control mussels. The visible light absorption spectrum revealed a pattern of absorptions that are characteristic of phytoplankton pigments. An initial examination of the toxic mussels revealed that they were engorged with green plankton, while the nontoxic mussels weren't. This was an important clue in the search for the origins of the toxin.
revealed a pattern of absorptions that are characteristic of phytoplankton pigments. An initial examination of the toxic mussels revealed that they were engorged with green plankton, while the nontoxic mussels weren't. This was an important clue in the search for the origins of the toxin.
But the pigments themselves were not poisonous. The dichloromethane fraction gave a negative result in the mouse bioassay. The aqueous layer contained the toxin, indicating that it was probably a polar, ionizable substance. This was a lucky break, because the researchers could discard the complex dichloromethane fraction and concentrate on the much simpler aqueous fraction.
Separation by polarity
Column chromatography was used to separate the aqueous layer into simpler components. The sample was passed through a narrow tube packed with beads of a resin called XAD-2, which grabs the nonpolar parts of passing molecules, but lets ions pass freely.
was used to separate the aqueous layer into simpler components. The sample was passed through a narrow tube packed with beads of a resin called XAD-2, which grabs the nonpolar parts of passing molecules, but lets ions pass freely. XAD-2 chromatography is particulary effective for separating organic acids and bases. Flushing the resin with a strong base ionizes acids in the sample. The ionized acids will pass through the column before other organic compounds because the resin won't retain them in their polar ionized form. Flushing with a strong acid gives organic bases in the sample extra hydrogen ions (and a positive charge); any organic bases adsorbed onto the resin will be washed out of the column.
Of the many fractions that passed out of the XAD-2 column, only one was toxic. For the final stage of the purification, the toxic fraction was separated with high performance liquid chromatography (HPLC). Again, a polar solution containing the sample was passed through a column packed with a nonpolar stationary phase
(HPLC). Again, a polar solution containing the sample was passed through a column packed with a nonpolar stationary phase . A single, highly purified fraction collected from the HPLC column accounted for all of the toxicity present in the original mussel sample. The toxin was isolated.
. A single, highly purified fraction collected from the HPLC column accounted for all of the toxicity present in the original mussel sample. The toxin was isolated.
Separation by charge, size, and molecular shape
The researchers had to ensure that the final HPLC fraction was indeed the isolated toxic component. They separated the aqueous XAD-2 fraction again, using a completely different technique: high voltage paper electrophoresis.Electrophoresis is a technique for separating ions based on their charge-to-mass ratios. Ions placed between a positive and a negative electrode will move towards the electrode with the opposite charge. Generally, the higher the ion's charge-to-mass ratio, the faster it moves towards the electrode. The smallest and most highly charged ions move ahead of larger ions with lower charges. Molecular shape also affects the rate of migration; shapes with more concentrated charges tend to migrate faster, all other things being equal.
The sample is applied to a piece of blotting paper. The ends of the paper were dipped in pH buffer solutions; an electrode was placed in each buffer solution. Ions for separate substances migrate at different rates and were resolved as separate bands across the paper. A developing agent (ninhydrin) was sprayed on the paper to stain the bands to make them easier to see.
A band very close to the band for glutamic acid was observed in the electrophoresis of the toxic XAD-2 fraction, but not in the control fraction. It stained a distinctly different color from the glutamic acid. When the material in the band was collected and injected onto the HPLC column, it took exactly the same amount of time to move through the column as the toxic component found by the HPLC analysis. It also produced exactly the same amount of toxicity as the HPLC fraction had.
was observed in the electrophoresis of the toxic XAD-2 fraction, but not in the control fraction. It stained a distinctly different color from the glutamic acid. When the material in the band was collected and injected onto the HPLC column, it took exactly the same amount of time to move through the column as the toxic component found by the HPLC analysis. It also produced exactly the same amount of toxicity as the HPLC fraction had.
Identification of the toxin
Mass spectrometry was used to determine the compound's molecular weight (312 g/mol) and molecular formula (C15H22NO6). Spectroscopic analysis revealed the presence of conjugated double bonds and features characteristic of an amino acid
was used to determine the compound's molecular weight (312 g/mol) and molecular formula (C15H22NO6). Spectroscopic analysis revealed the presence of conjugated double bonds and features characteristic of an amino acid . By matching the spectra with those from STN International's Registry system, the compound was unambiguously identified as domoic acid, an triprotic amino acid:
. By matching the spectra with those from STN International's Registry system, the compound was unambiguously identified as domoic acid, an triprotic amino acid: 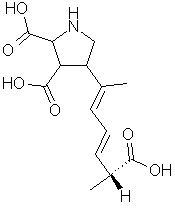 | 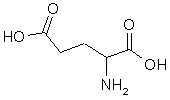 | ||
| Domoic acid in acidic solution. | Glutamic acid in acidic solution. | ||
| Click the images for 3D Chime structures. | |||
Some scientists insisted that domoic acid could not be responsible for the poisonings, because it had been used as a folk remedy for intestinal worms in Japan for many years. There were no previous reports of toxicity from seaweed or seaweed extracts known to contain domoic acid in the medical literature. However, the seaweed extracts used in the remedies contained a total dose of no more than 20 mg of domoic acid, while some of the victims of amnesic shellfish poisoning consumed some 290 mg [Perl]. Many substances that are harmless or even beneficial at low dosages can have toxic effects at higher levels.
Domoic acid is a molecular Trojan Horse. Nerve cells mistakenly recognize domoic acid as glutamic acid - a fatal error. Glutamate (the ionized form of glutamic acid) is a neurotransmitter
- a fatal error. Glutamate (the ionized form of glutamic acid) is a neurotransmitter , a molecule used to send a message from one nerve cell to another. When the glutamate molecule binds to a glutamate receptor
, a molecule used to send a message from one nerve cell to another. When the glutamate molecule binds to a glutamate receptor embedded on the membrane of the receiving nerve cell, the receptor opens channels in the membrane that allow calcium ions to flow into the cell. The influx of charge causes a voltage to build up across the cell membrane, and the nerve cell fires, passing the signal on to the next nerve cell. Frequent stimulation can cause new connections to grow between the neurons, so glutamate plays a fundamental role in thought, learning and memory.
embedded on the membrane of the receiving nerve cell, the receptor opens channels in the membrane that allow calcium ions to flow into the cell. The influx of charge causes a voltage to build up across the cell membrane, and the nerve cell fires, passing the signal on to the next nerve cell. Frequent stimulation can cause new connections to grow between the neurons, so glutamate plays a fundamental role in thought, learning and memory.
It is possible to have too much of a good thing, however. Glutamate at high concentrations acts as an excitotoxin -a compound that kills cells by literally exciting them to death. Excess glutamate keeps the gates that allow calcium ions across the cell membrane open too long. Calcium ions flood into the cell, causing it fire uncontrollably. The neuron swells and eventually bursts. The damage cascades to nearby neurons because the damaged and ruptured neurons release their glutamate and other excitatory amino acids, overstimulating nearby cells. The excess calcium inside the cell stimulates certain protein-cutting enzymes, which produce large quantities of free radicals
-a compound that kills cells by literally exciting them to death. Excess glutamate keeps the gates that allow calcium ions across the cell membrane open too long. Calcium ions flood into the cell, causing it fire uncontrollably. The neuron swells and eventually bursts. The damage cascades to nearby neurons because the damaged and ruptured neurons release their glutamate and other excitatory amino acids, overstimulating nearby cells. The excess calcium inside the cell stimulates certain protein-cutting enzymes, which produce large quantities of free radicals as a by-product. The free radicals are extremely reactive, and damage any biochemical structure they come into contact with [Berman]. This excitotoxic cascade is thought to play an important role in brain injury and neurodegenerative diseases.
as a by-product. The free radicals are extremely reactive, and damage any biochemical structure they come into contact with [Berman]. This excitotoxic cascade is thought to play an important role in brain injury and neurodegenerative diseases.
Domoic acid's structure is obviously similar to glutamic acid. But its five-sided ring makes it less flexible than glutamate, which causes it to bind very tightly to glutamate receptors. As a result, the excitatory effect of domoate is 30 to 100 times more powerful than that of glutamate [Perl].
How did the domoic acid get into the shellfish (and the anchovies eaten by the birds at Capitola)? Remember that phytoplankton pigments were found in the aqueous layer after solvent extraction. This wasn't quite a smoking gun, but it was definitely a fingerprint of the killer. An extensive investigation traced the domic acid to an obscure species of needle-like diatom*, called Pseudo-nitzschia pungens (shown in the title banner at the top of this page). Pseudo-nitzschia has been found in oceans around the world, so further outbreaks are possible in many locations. Commercial shellfish and seafood is now monitored regularly for domoic acid, using HPLC to identify the toxin. The screening and testing procedures have so far been successful- not a single instance of domoic acid poisoning in humans has been reported since the 1987 outbreak.
References and Links
Accounts of domoic acid outbreaks
- Hitch's Birds Deranged by Dodgy Anchovies (New Scientist)
- An article describing the 1961, 1987, and 1991 domoic acid poisonings, by Rosie Mestel (p. 6, July 22, 1995)
- Diatoms Nature's Marble: Hazard (Eureka)
- A brief description with electron micrographs of the diatoms that produce domoic acid. The 1991 incident of domoic acid poisoning in sea birds around Monterrey Bay, California is described.
- An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. (Perl, et. al., Health and Welfare Canada)
- The field epidemiologists who investigated the original 1987 outbreak report their findings. The destruction of short-term memory experienced by some of the poisoning victims is described.
Perl, T.M., L. Bard, T. Kosatsky, J.C. Hockin, E. Todd, and R.S. Remis, New England J. Med. 322: 1775-1780 (1990). - Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels (Montreal Neurological Institute)
- A clinical description of the 1987 outbreak of amnesic shellfish poisoning on Prince Edward Island.
J. S. Teitelbaum, R. J. Zatorre, S. Carpenter, D. Gendron, A. C. Evans, A. Gjedde, N. R. Cashman, New England J. Med. 322: 1781-1787 (1990).
Domoic acid chemistry
- Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. (Atlantic Research Laboratory, Department of Fisheries and Oceans)
- Details of the analytical procedure used to identify the culprit in amnesic shellfish poisoning.
Wright, J.L.C., R.K. Boyd, A.S.W. de Freitas, M. Falk, R.A. Foxall, W.D. Jamieson, M. V. Laycock, A.W. McCulloch, A.G. McInnes, P. Odense, V. Pathak, M.A. Quilliam, M.A. Ragan, P.G. Sim, P. Thibault, J.A. Walter, M. Gilgan, D.J.A. Richard, and D. Dewar, Canadian Journal of Chemistry, 67 481-490 (1989). - The amnesic shellfish poisoning mystery (M. A. Quilliam, J. L. C. Wright)
- A retrospective account of the analytical approach for the first isolation of domoic acid from the toxic mussels.
M. A. Quilliam, J. L. C. Wright, Analytical Chemistry, 61 (18) 1053A-1060A, 1989. - Domoic Acid and Pseudo-Nitzschia References (Stephen Bates, Dept. of Fisheries and Oceans, Gulf Fisheries Centre)
- An extensive bibliography about domoic acid and the organisms that produce it.
- Marine Biotoxins Chemical Structures (National Oceanic and Atmospheric Administration)
- Part of NOAA's Harmful Algae Bloom site, this page discusses the structure and biological activity of domoic acid and the saxitoxins, which cause paralytic shellfish poisoning.
- Receptor Binding Assays and Marine Biotoxins (National Oceanic and Atmospheric Administration)
- Domoic acid's toxicity stems from its ability to bind tightly to glutamate receptors. Scientists at the Northwest Fisheries Science Center at NOAA have used domoic acid's ability to displace kainic acid from the receptor to developed a very sensitive technique for detecting domoic acid in water samples.
Glutamate and domoic acid pharmacology
- Domoic Acid Toxicity in Cerebellar Granule Neurons (Journal of Neurochemistry)
- Domoic acid kills nerve cells by causing them to fire until they die. The toxin works by causing the affected cells to uncontrollably release excitatory amino acids that influence nearby cells. The details may be found in this paper by two researchers at the College of Pharmacy and Toxicology Program at Oregon State University.
- F. W. Berman, T. F. Murray , J. Neurochem. 69, 693-703 (1997).





Comments :
Post a Comment